Clinical evidence
RegenRPR in several tendinopathies
RegenPRP has been evaluated for several tendinopathies including:
- Chronic tendinopathies
- Non-insertional tendinopathies compared to enthesopathies
- Gluteus tendon
- Shoulder supraspinatus
- Foot plantar fascia
- Specific research:
- Achilles and patellar tendinopathies
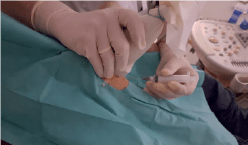
Figure 1: Ultrasound controlled injection of RegenPRP in Achilles tendinopathies.
Achilles tendinopathies
Objective:
Evaluate the use of RegenPRP for the treatment of recalcitrant non-insertional Achilles tendinopathies and investigate whether positive outcomes
depend on the age of the patients.3
Protocol:
Retrospective study on 44 patients who had
failed to respond to conservative treatment.
Patients received a total of 3 treatments at weekly intervals. RegenPRP (~ 4 ml) was injected at several sites into the degenerated tendon area. Injections were performed in sterile conditions, without anaesthesia and under ultrasound (US) control (Fig. 1).
Functional evaluation of Achilles tendon was performed using Victorian Institute of Sports Assessment-Achilles questionnaire (VISA-A) at baseline and at 1, 3, 6 and 12 months after the treatment. Patients were divided according to their age in two cohorts (Young <55 years old; Elderly >55 years old) (Fig. 2).
RESULTS
- A constant and significant increase of the VISA-A score was observed for both patient groups during the whole length of the follow up, although better outcomes were seen in younger patients.
- Improvement of Achilles tendon functionality after RegenPRP treatment was sustained and significant for 12 months.
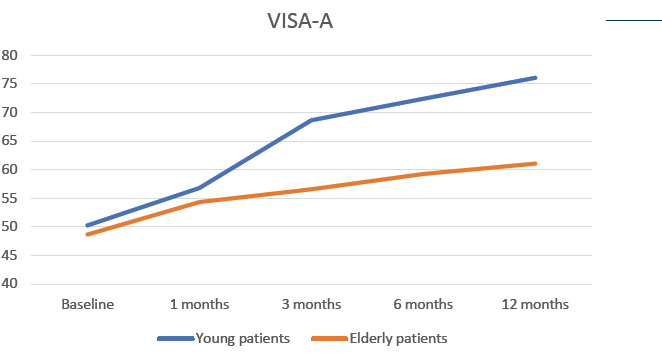
Figure 2: Evolution of VISA-A score in patients treated with RegenPRP, stratified according to their age group.
CONCLUSION
When injected into tendon lesions, RegenPRP showed good outcomes in patients suffering from recalcitrant Achilles non-insertional tendinopathies.
Patellar tendinopathies
Objective:
Examine the effect of RegenPRP in chronic
Achilles and patellar tendinopathies.4
Protocol:
Case series study on 21 patients suffering from chronic tendinopathy for at least 6 months: 14 cases of Achilles tendinopathy and 7 cases of patellar tendinopathy. All patients were sportspeople not responding to conventional treatments.
Patients received a total of 3 infiltrations at weekly intervals. RegenPRP was injected half in the tendon lesion and half in the peritendinous area under US control with local anaesthesia. In addition, patients followed a personalized rehabilitation programme for one month.
Functional evaluation was performed using the VISA questionnaire for Achilles (VISA-A) and patellar tendon (VISA-P) at baseline, at the end of the treatment cycle (3 weeks) and at 12- and 24-months follow-up (figure 3). A numeric rating scale was also used to measure subjective pain.
RESULTS
- A significant improvement of the VISA scores was observed at the end of the treatment cycle at 3 weeks. The benefice was sustained all along the two years of follow-up period (Fig. 3) and accompanied by a significant reduction in pain (p<0.01).
- Ultrasound scans performed at the end of the follow up showed a visible reduction in tissue irregularity in 86% of infiltrated tendons.
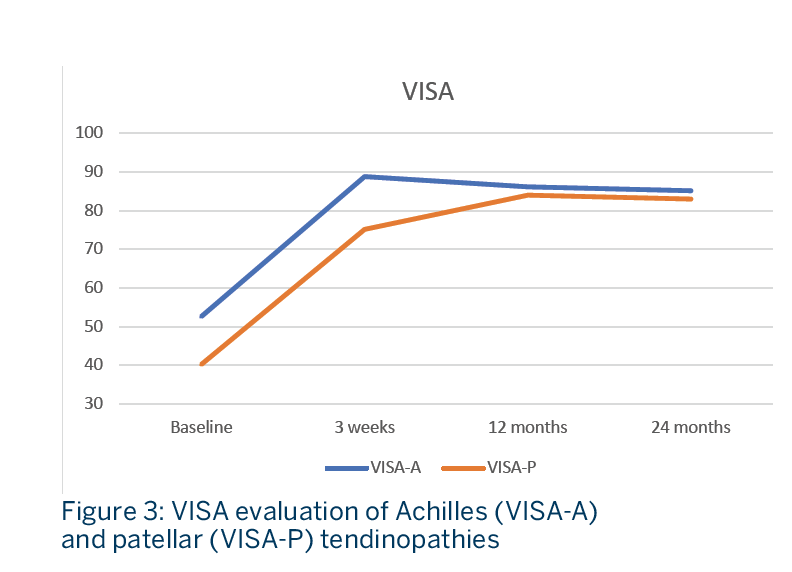
CONCLUSION
At the end of the follow-up, patients demonstrated a complete functional recovery and no longer complained of pain. In addition, RegenPRP treatment was well tolerated and without adverse events.
Elbow tendinopathies
Objective:
Examine the effect of RegenPRP in twenty-two elbows with tendinitis (epicondylitis n=19 and chronic tricipital tendinitis n=3) after failure with at least two usual treatments.5
Protocol:
Case series study on 22 patients with elbow tendinopathy not responding to conventional treatments for 3 to 18 months. Ten patients received
1 injection, 9 patients received 2 injections and 3 patients received 3 injections of RegenPRP.
PRP was injected intra-tendinously by peppering (point-by-point and nappage technique), without local anaesthesia.
Evaluation of pain was performed one- and two-months after the last injection. Follow up examination was performed after 9 to 22 months.
RESULTS
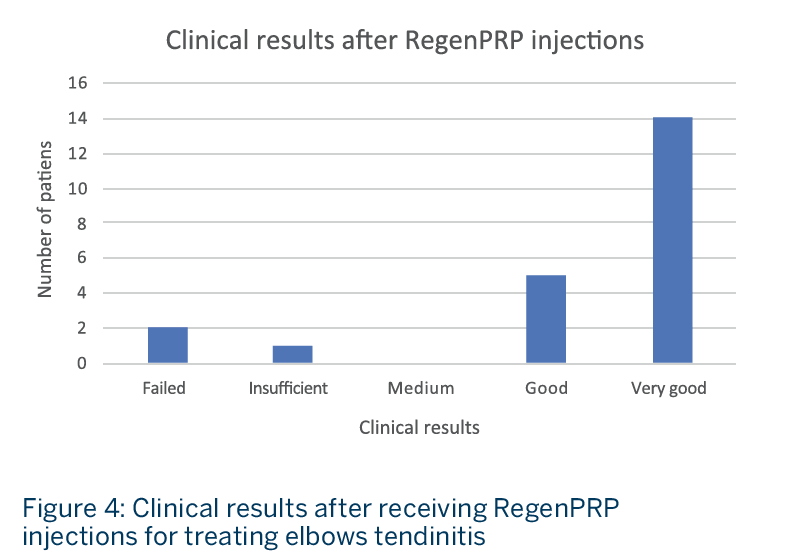
- Nineteen patients (86%) presented good (5 patients) or very good (14 patients) results after receiving RegenPRP treatment (Fig. 4).
- No relapse was seen over time and patients rated “good” at follow-up became “very good” over time.
- No adverse events were reported apart from transient local pain during or after PRP injection.
CONCLUSION
RegenPRP treatment resulted in long-term improvement of elbow tendinopathies not responding to conventional treatment.
Our products
Bibliography
- Fountain, John H., and Sarah L. Lappin. Physiology, Platelet. StatPearlsPublishing, Treasure Island (FL), 2019.
- Mathew, Joscilin, and Matthew Varacallo. Physiology, Blood Plasma. StatPearlsPublishing, Treasure Island (FL), 2019.
- Platelet-Rich Plasma therapy in non-insertional Achilles tendinopathies: The efficacy is reduced in 60-years old people compared to young and middle-age individuals. Salini V, Vanni D, Pantalone A, Abate M. Front Aging Neurosci 2015 ;7 :228.
- Crescibene, A., Napolitano, M., Sbano, R., Costabile, E., and Almolla, H. (2015). Infiltration of Autologous Growth Factors in Chronic Tendinopathies. Journal of blood transfusion 2015, 924380.
- Le Coz, J. (2011). Traitement de 22 cas de tendinites du coude, rebelles aux traitements classiques, par injection de plasma riche en plaquettes (PRP). Journal de Traumatologie du Sport 28, 83-89.
Contact Regenlab
Now is the time to change your patient’s life. Browse ressources and
get connected to treatment that is right for them.
For more information on our products, please contact us by clicking on the button below:
Our Regional offices are
located in
- New York (USA)
- Montréal (Canada)
- Venice (Italy)
- Munich (Germany)
- Paris (France)
- Dubai (U.A.E.)
- Beijing (China)
- Istanbul (Turkey)




