
Androgenetic alopecia
Ho A, Sukhdeo K, Lo Sicco K, Shapiro J.Trichologic response of platelet-rich plasma in androgenetic alopecia is maintained during combination therapy.
OBJECTIVE
Evaluate the efficacy and safety of RegenPRP™ mesotherapy in combination with other hair restorative modalities for the treatment of androgenetic alopecia.
PROTOCOL
Retrospective study
Twenty-four patients (5 women and 19 men, 23–73 years old) with androgenic alopecia and using concomitant hair restoration therapies were included in this study. All patients used topical 5% minoxidil and 83.3% (20/24) also used antiandrogen medications.
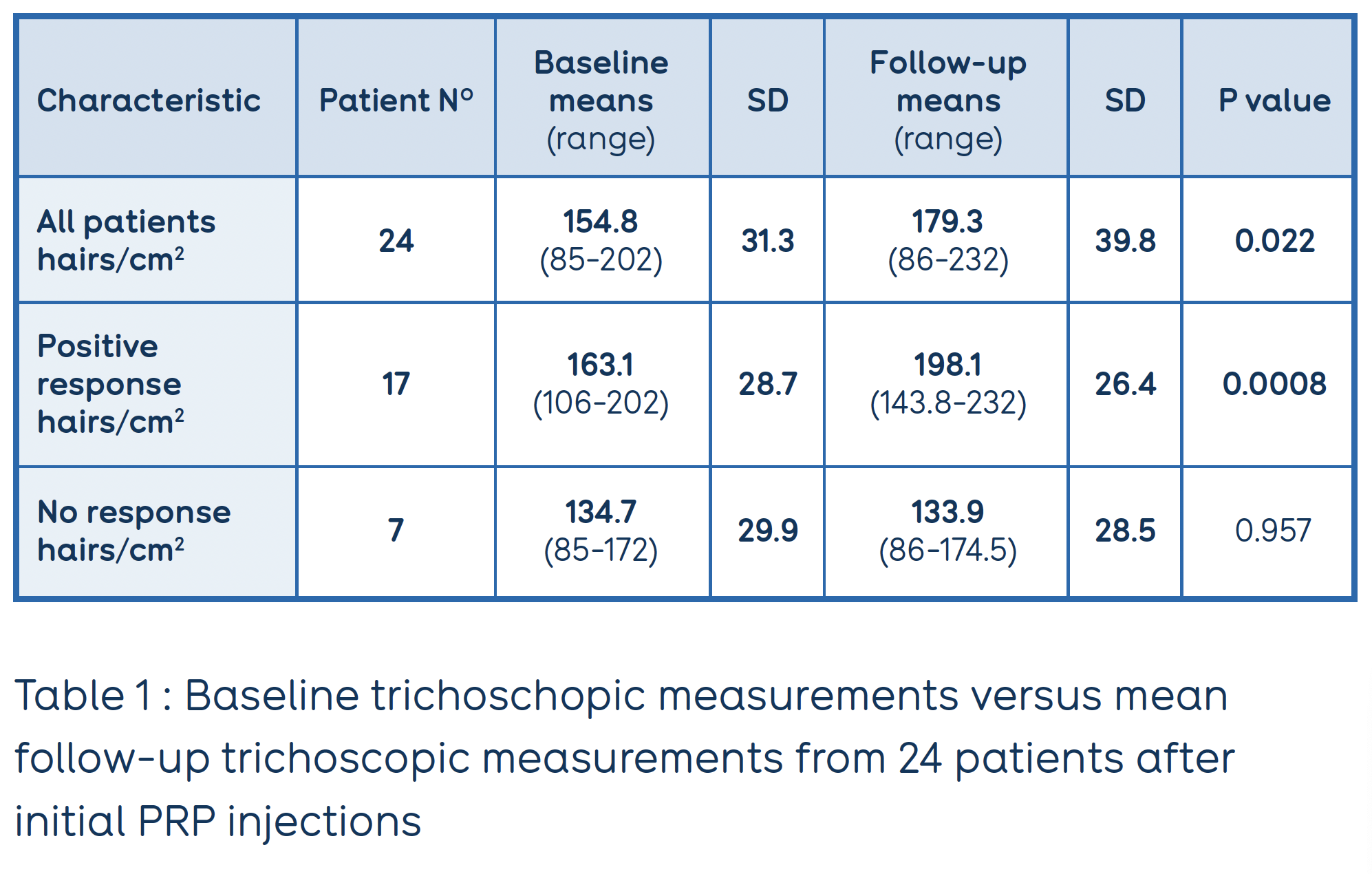
The treatment consisted of a monthly injection of RegenPRP™ for 6 months followed by injections every 3-6 months. Mean trichoscopic measurements of hair density and diameter throughout the treatment course (average 6 months; range 2-24 months) were compared to baseline measures.
RESULTS
A positive response to RegenPRP™ was seen in 70.8% (17/24) of patients, as early as 2 months after the initial injections. Overall, mean hair density after RegenPRP™ showed a statistically significant increase from baseline on the anterior crown (124.5 hairs/cm2, p=0.022 ; Table 1).
Hair density with RegenPRP™ increased > 10% over baseline in 62.5% (15/24) of patients and >20% over baseline in 33.3% (8/24) of patients. One patient had an increase in hair density of >50%.
RegenPRP™ can be used successfully in combination with other hair restoration modalities such as minoxidil and finasteride.
CONCLUSION
RegenPRP™ as combination therapy demonstrated a significant increase in density of hairs.
REFERENCE
Ho A, Sukhdeo K, Lo Sicco K, Shapiro Trichologic response of platelet-rich plasma in androgenetic alopecia is maintained during combination therapy. J Am Acad Dermatol 2020;82(2):478-9.
