
FEMALE SEXUAL DYSFUNCTION
Runels 2014A Pilot Study of the Effect of Localized Injections of Autologous Platelet Rich Plasma (PRP) for the Treatment of Female Sexual Dysfunction.
Female sexual dysfunction may be manifested by decreases in sexual desire, arousal and lubrication, leading to dyspareunia.
OBJECTIVE
Evaluate the response of women with varying degrees of sexual dysfunction to treatment with RegenPRP™.
PROTOCOL
Case series study on 11 women (aged 24-64 years old) presenting symptoms of sexual dysfunction such as hyposexual desire disorder, arousal disorder, orgasmic disorder, and dyspareunia. RegenPRP™ was injected into the anterior vaginal wall, into a space between vagina and urethra most distal from bladder, and into the clitoris. Two standardized sexuality tests, the Female Sexual Function Index (FSFI) and the Female Sexual Distress Scale (FSDS), were used to measure the response to this therapeutic intervention.
RESULTS
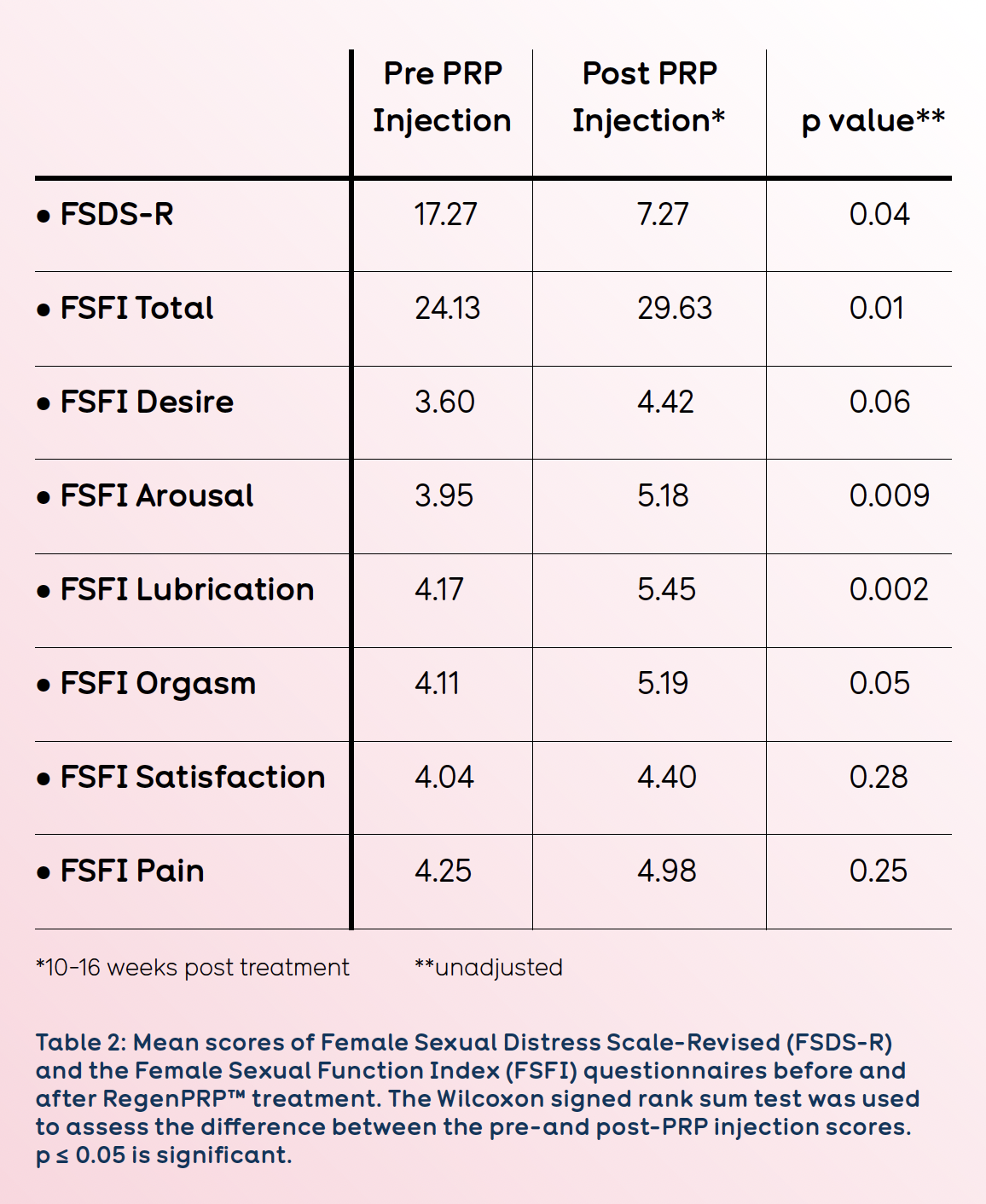
Of the 11 patients treated, seven (64%) demonstrated some degree of improvement. The mean FSDS revised score dropped from 17.27 before treatment to 7.27 at 12-16 weeks postinjection. Five of the 7 patients who had a FSDS score over the threshold value of 11 before treatment had a post treatment score below 7. Evaluations based on FSFI score indicated an overall significant improvement after injection.
The scores from the FSFI subscales of desire, arousal, lubrication, and orgasm were all significantly increased after injection of RegenPRP™ (P ≤ 0.05). The changes in the scores for satisfaction and pain were considered not significant (See Table 2).
CONCLUSION
The results of this pilot study suggest that RegenPRP™ injections is effective for the treatment of certain types of female sexual dysfunction.
Significant improvement of the FSFI score was observed after RegenPRP™ injection, especially for desire, arousal, lubrication, and orgasm.
REFERENCE
Runels C. A Pilot Study of the Effect of Localized Injections of Autologous Platelet Rich Plasma (PRP) for the Treatment of Female Sexual Dysfunction. Journal of Women’s Health Care 2014;03(04).
