
ACNE & KELOID SCARS
ACNE SCARS
Application of regenerative medicine in treatment of acne scars.
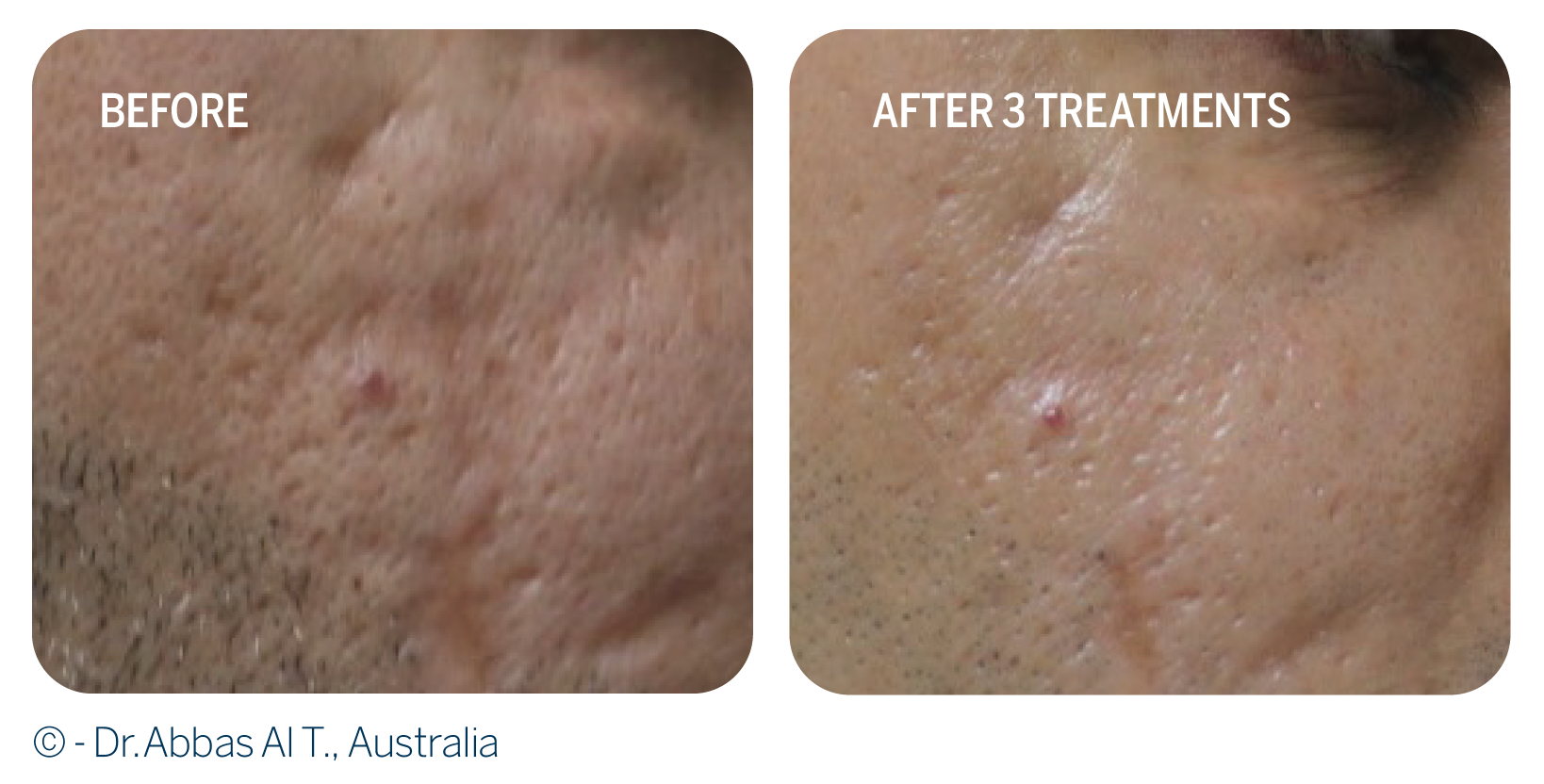
The efficacy of a nanofat and plasma rich-platlets injection and fractional CO2 laser resurfacing was evaluated in 30 patients. • The authors showed that the combined approach of nanofat and RegenPRP injection and CO2 laser can improve the appearance of atrophic scars. RegenPRP and nanofat injection also significantly increased skin and subcutaneous tissue thickness.
• Patients reported great satisfaction with the treatment and confirmed the impact of facial acne scars on social life and relationships.
KELOID SCARS
Injecting platelet-rich plasma is an effective and safe method as an adjunctive therapy to resection for treating keloid scars refractory to conventional therapy.
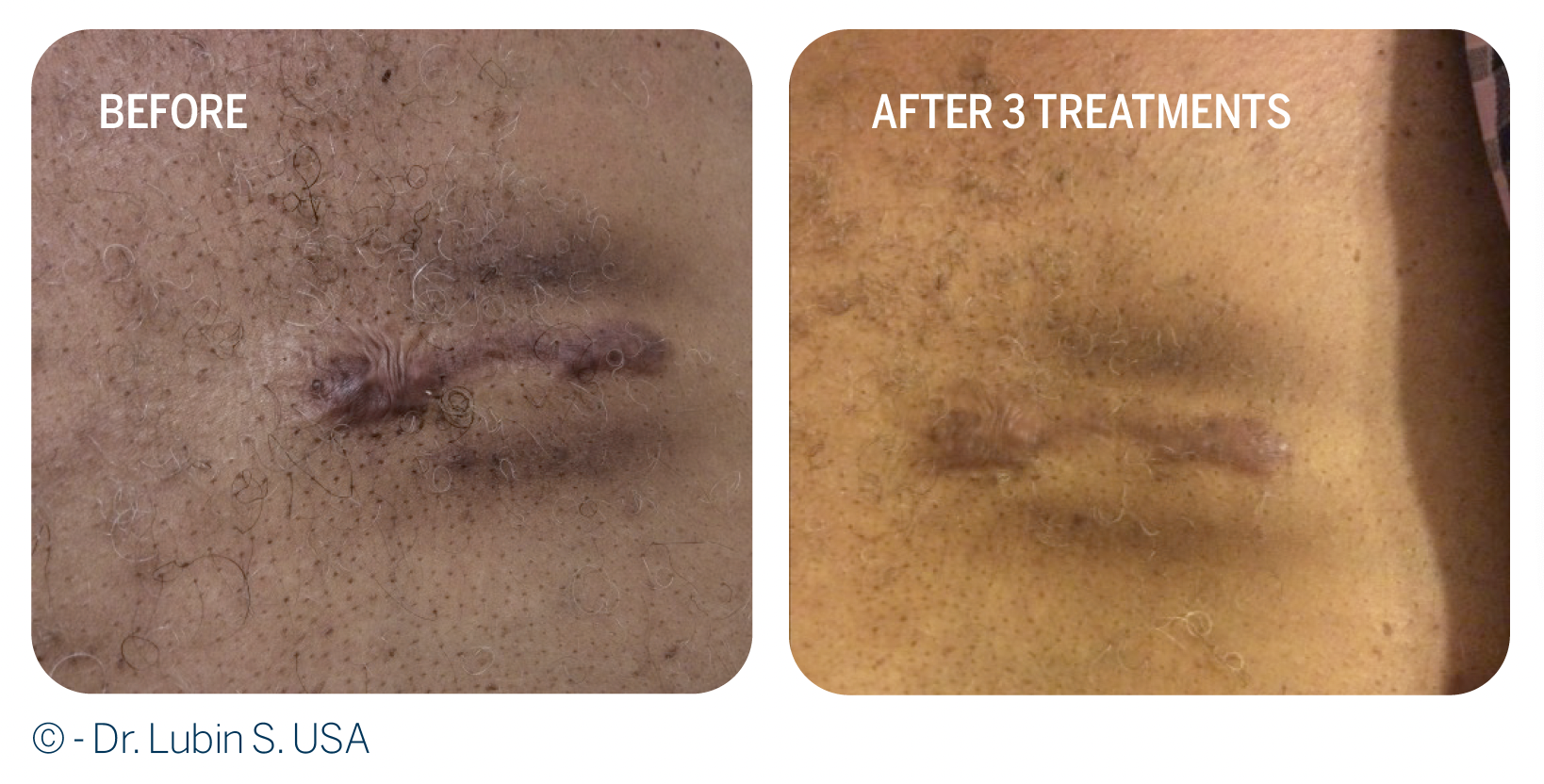
The efficacy of RegenPRP as a treatment of keloid scars was evaluated in 17 patients.7 Evaluations were based on the complete remission of the keloid scars, pruritus severity score and the mean Vancouver Scar Scale (VSS) score, two years after treatment with RegenPRP.
• 53% of keloid scars completely resolved at 2 years, and only 29% relapsed following RegenPRP treatment.
• Pruritus severity score was significantly lower at 2 years compared with baseline Pruritus symptom associated with keloid scar disappeared in 60% of patients.
• The VSS score, which assesses 4 variables: vascularity, height/thickness, pliability, and pigmentation, was significantly improved at the 2 year follow-up (p < 0.001) and had improved in 88% of patients.
REFERENCE
6 Tenna, S., et al., Comparative Study Using Autologous Fat Grafts Plus Platelet-Rich Plasma With or Without Fractional CO2 Laser Resurfacing in Treatment of Acne Scars: Analysis of Outcomes and Satisfaction With FACE-Q. Aesthetic Plast Surg, 2017. 41(3): p. 661-666.
7 Hersant, B., et al., Efficacy of Autologous Platelet Concentrates as Adjuvant Therapy to Surgical Excision in the Treatment of Keloid Scars Refractory to Conventional Treatments: A Pilot Prospective Study. Ann Plast Surg, 2018. 81(2): p. 170-175.
