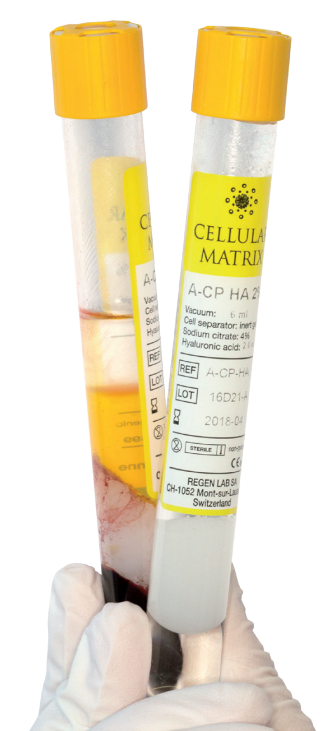
What is Cellular Matrix® ?
Cellular Matrix® tubes allow the preparation of autologous platelet-rich plasma (RegenPRP®) combined with a non-crosslinked hyaluronic acid (HA) in a closed-circuit system.
CM-PRP-HA has an excellent safety profile in clinical practice.1,3,4
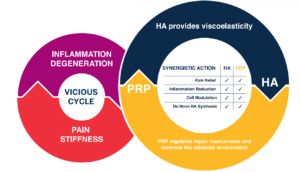
Cellular Matrix® technology combines the complementary clinical effects of RegenPRP® and hyaluronic acid (HA), giving better and longer-lasting results in patients with osteoarthritis.1,3,4
Hyaluronic Acid
Hyaluronic acid is a major component of synovial fluid contributing to joint homeostasis.
- 25 years of clinical experience shows pain relief and functional improvement lasting 6 to 12 months in OA patients.
- Plays a major role in viscosupplementation and pain relief in OA.5
- The network of HA chains generates an ideal cell-friendly matrix when combined with PRP.6
Hyaluronic acid improves the activity of several molecules contained in platelet-rich plasma to provide additional benefits to OA patients1.
Both PRP and HA have been extensively used to improve lubrication, modulate inflammation and modify the joint catabolic micro-environment, aiming not only to reduce clinical symptoms, but also to slow down OA progression2.
When PRP and HA are used in combination these effects are enhanced and prolonged. HA creates a bioactive scaffolding in which the platelets progressively release their growth factors. RegenPRP does not negatively affect the mechanical, elastic or viscous properties of HA1.
Technology platform for standardized autologous regenerative medicine
The simple, safe and efficient point-of-care preparation of autologous platelet-rich plasma.
 Blood Collection
Blood Collection
 Centrifugation
Centrifugation
 Resuspension
Resuspension
 Resuspended
Resuspended
 Ready to Use
Ready to Use
TECHNOLOGY ADVANTAGES
- User-independent standardized preparation
- Minimum volume of blood required
- Safe closed-circuit system
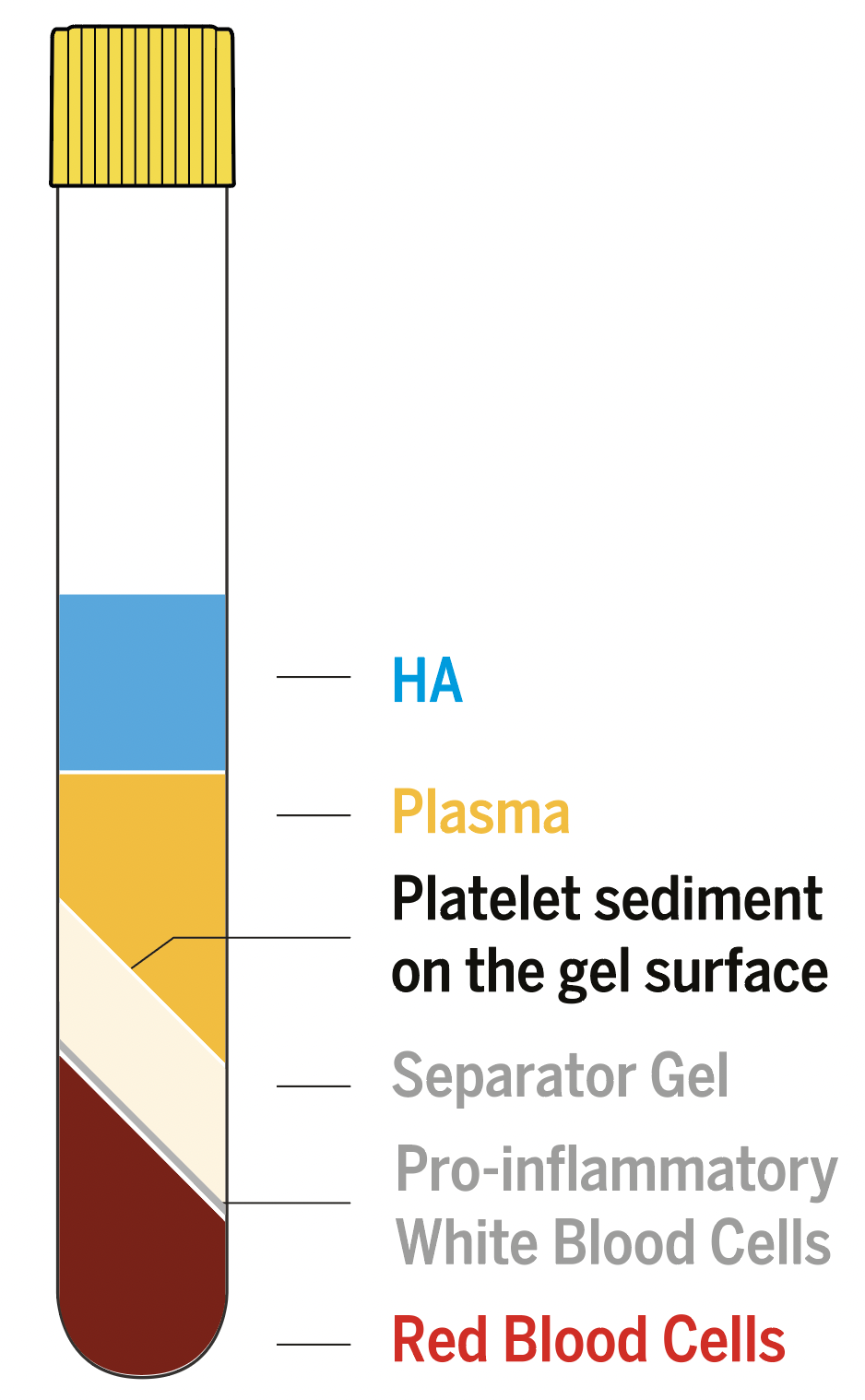
- Mechanical separation of PRP using a chemically inert gel after a 5-minute centrifugation at 1500g.
- Pharmaceutical grade solution of sodium citrate allowing a reversible anticoagulation
- Minimal learning curve and ease of use
- Operationally and clinically efficient process
- Facilitates and streamlines routine practice
CELLULAR MATRIX PLATFORM
- Certified technology for the preparation of RegenPRP® combined with hyaluronic acid (HA) (CM-PRP-HA)
- Contains 2 ml of non-crosslinked HA at a concentration of 20 mg/ml (40 mg total)
- HA produced by bacterial fermentation, thus free of animal proteins
SCIENTIFIC ADVANTAGES OF RegenPRP®
- Demonstrated safety and efficacy
- Evidence-based outcomes for numerous therapeutic indications
- Large number of clinical studies, with over 200 publications
Biological advantages
- RegenPRP® is standardized, leucocyte reduced and easily reproducible
Regen Lab specific separating gel technology guarantees minimal variability - Platelets recovery in CM-PRP-HA >70%
- High platelet quality
Viable & functional platelets - Full plasma recovery
No loss of plasma growth factors and fibrinogen - Leucocyte reduced PRP Depletion ~ 94.3% of pro-inflammatory granulocytes, leaving mainly lymphocytes and monocytes
- Virtually no red blood cells
Depletion of ~ 99.5% of erythrocytes
RegenPRP standardized performance
A-CP-HA Tube Properties

BLOOD SAMPLE VOL PER TUBE
6 ml

PRP VOL PER TUBE
~3 ml of PRP combined with 2 ml of HA

PLATELET RECOVERY
> 70 %

GRANULOCYTE DEPLETION
94.3 %

RED BLOOD CELL DEPLETION
99.5 %
Our products
Clinical evidence
Intended use of the device
Device used to prepare a combination of hyaluronic acid (HA) and platelet rich plasma (PRP) for intra-articular injections for symptomatic treatment of pain and mobility improvement in knee osteoarthritis

Cellular Matrix / A-CP-HA-1
Ref: A-CP-HA-1
1 A-CP-HA tubes
An implant card will be supplied with the device,
one for each A-CP-HA tube. This implant card is intended
to be filled in by you with the patient’s information
after the medical procedure. You shall give this card
to the patient, who will keep it for at least 30 days
Cellular Matrix / A-CP-HA-3
Ref: A-CP-HA-3
3 A-CP-HA tubes
These are medical devices. Please use them according to the instructions for use.


Contraindications
Absolute contraindications:
- Platelet dysfunction syndrome
- Critical thrombocytopenia
- Hemodynamic instability
- Severe metabolic or systemic disorders
- Septicemia
- Acute/local infection at the site of the procedure
- Patient unwilling to accept risks
- Hypersensitivity to one of the components (including HA)
Relative contraindications:
- Consistent use of NSAIDs within 48 hours before the procedure
- Consistent use of other medication(s) or dietary supplement(s) which alter platelet function, within 3 days before the procedure
- Corticosteroid injection at the treatment site within 1 month before the procedure
- Systemic use of corticosteroids within 2 weeks before the procedure
- Tobacco use
- Recent fever or illness
- Malignant diseases, especially those affecting blood, bone marrow or bones, and cancers in metastatic phase
- Autoimmune diseases with presence of antibodies and progressive (Hashimoto, rheumatoid arthritis, lupus, etc.)
- Impaired coagulation
- Hemoglobin count < 10 g/dl
- Platelet count < 105/ µl
Possible side effects
Possible side effects of blood collection:
Blood collection may cause damage to the blood vessels, hematomas, superficial phlebitis, early or late infection and/or temporary or permanent nerve damage that may result in pain or numbness.
Possible side effects of intra-articular injections
Following intra-articular injections, local secondary inflammatory reactions may occur at the site of injection. This may result in temporary pain, feeling of heat, redness and swelling in the joint area treated with the PRP/HA preparation. Use of ice packs in the minutes following the injection, or oral analgesic treatment (acetaminophen) the day following the injection may reduce these effects. The intake of non-steroidal anti-inflammatory drugs (NSAID) must be avoided. Following injection with HA, there have also been occasional reports of hyper-sensitivity, including, rarely, anaphylaxis. The administration of HA was also reported to provoke pronounced inflammatory reactions. Injection may lead to infection if general precautions for injection and asepsis are not respected. NB: In case any serious incident occurs in relation to the device, the user and/or patient should report this incident to the manufacturer and the competent authority of the Member State in which the user and/or patient is established.
Precautions
Intra-articular injection precautions
Injections of the PRP/HA combination into the joint cavity must be performed with the same precautions as any other intra-articular injection, and preferably using ultrasound guidance. Joint effusion, if present, should be removed before injecting the PRP/HA combination. The physician must evaluate the physical aspect of the collected fluid. If any doubt, appropriate measures should be undertaken, and the physician must assess whether the intra-articular injection of PRP/HA should be performed or not. Following intra-articular injection, the patient must rest for 1h after the injection (no physical activity) and avoid prolonged (no more than 10 minutes) standing or walking for the first 12 hours after the intra-articular injection.
General precautions
The patient must be informed about the general risks associated with the treatment and the possible adverse effects. Before use, the physician must consider the patient’s medical history, especially in terms of coagulation disorders, and in case of doubt blood platelet count must be performed. When a definitive diagnosis cannot be established, and the conditions listed as contraindicated cannot be confidently excluded, the physician must perform an appropriate benefit to risk assessment for the patient prior to using the device. Strict aseptic technique must be followed during phlebotomy and injection. Use proper safety precautions to avoid contact with patient blood or cross-contamination. Disinfectants containing quaternary ammonium salts should not be used to prepare the skin for injection because hyaluronan can precipitate in their presence. The PRP/ HA combination must be prepared from fresh blood and must be used within four hours (extemporaneous use only) by a qualified physician. Use proper safety precautions to protect against needles or broken tubes. Do not re-cap the needle after use and discard directly in the biological hazard container. Do not use the sterile components of this kit if the blister pack is opened or damaged. Do not use the components of this kit if they are broken or have a defect. Do not use the tube if it has lost vacuum. Do not use the sodium citrate solution or other tube components separately. Store between 5 °C and 30 °C; bring the kit to ambient temperature before using the tubes. Each Cellular Matrix A-CP-HA tube is for single use only; do not reuse. Reuse may lead to infection or other illness / injury. Do not re-sterilize, do not use after the expiration date. To respect the closed-circuit procedure, the user should use the accessories for phlebotomy and PRP/ HA combination collection (provided separately in the Accessory Set). Tubes must not be opened. Standard needles with coring tips should not be used to pierce the tube stopper.
All tubes are to be discarded using the appropriate disposal method for potentially contaminated blood products. Do not inject the PRP/HA combination intravascularly or for any application other than those described in the intended use. Follow the manufacturer’s instructions when using the centrifuge. Use a fixed 45° angle head centrifuge (e.g., RegenPRP Centri provided by Regen Lab). As an alternative, a horizontal head (swinging bucket) centrifuge can be used. However, this would result in a small increase in red and white blood cell contamination in the resulting PRP/HA combination. Tubes should be centrifuged, as recommended in the instructions for use, at a relative centrifugal force (RCF) of 1500 g. Excessive RCF (over 2200 g) may lead to tube breakage resulting in blood exposure, and possible injury. RCF below 1500 g may lead to incorrect blood separation and red blood cell contamination of the PRP/HA combination. Centrifuge carriers and insert size should be adapted to the tubes. Use of carriers too large or too short may result in breakage of the tubes. Caution should be taken to ensure that tubes are properly seated in the centrifuge carriers. Tubes must be balanced in the centrifuge.
Patented by Regen Lab SA
Cellular Matrix (PRP+HA)
U.S. patent US8945537,
U.S. patent US9517255,
European patent EP2544697B1
Canadian patent CA2789533C,
Chinese patent CN103079577B,
Australian patent AU2011225828B,
Japanese patent JP6076091,
Russian patent RU2614722,
Israeli patent IL221133
Bibliography
- Abate M. et al. Efficacy and safety profile of a compound composed of platelet-rich plasma and hyaluronic acid in the treatment for knee osteoarthritis (preliminary results). Eur J Orthop Surg Traumatol 2015 Dec;25(8):1321-6.
- Seleem, N.A., et al., Intra-Articular Injections of Platelet-Rich Plasma Combined with Hyaluronic Acid Versus Hyaluronic Acid Alone in Treatment of Knee Osteoarthritis. ejpmr, 2017;4(4):608-15
- Renevier, J. L., et al. «Cellular matrix™ PRP-HA”: A new treatment option with platelet-rich plasma and hyaluronic acid for patients with osteoarthritis having had an unsatisfactory clinical response to hyaluronic acid alone: Results of a pilot, multicenter French study with long-term follow-up.» Int. J. Clin. Rheumatol. 2018;13(4):230-8
- Barac, B., et al. (The new treatment approach in knee osteoarthritis: Efficacy of cellular matrix combination of platelet rich plasma with hyaluronic acid versus two different types of hyaluronic acid (HA). Int. J. Clin. Rheumatol. 2018;13(5):289-95
- Huskisson, E. C., and S. Donnelly. «Hyaluronic Acid in the Treatment of Osteoarthritis of the Knee.» [In eng]. Rheumatology (Oxford) 38, no. 7 (Jul 1999): 602-7.
- Smith J.D. et al. Improved growth factor directed vascularisation into fibrin constructs through inclusion of additional extracellular molecules. Microvasc Res.
2007;73(2):84-94.
Contact Regenlab
Now is the time to change your patient’s life. Browse ressources and
get connected to treatment that is right for them.
For more information on our products, please contact us by clicking on the button below:
Our Regional offices are
located in
- New York (USA)
- Montréal (Canada)
- Venice (Italy)
- Munich (Germany)
- Paris (France)
- Dubai (U.A.E.)
- Beijing (China)
- Istanbul (Turkey)