
Achilles tendinopathies
Salini 2015Platelet Rich Plasma Therapy in Non-insertional Achilles Tendinopathy: The Eicacy is Reduced in 60-years Old People Compared to Young and Middle-Age Individuals.
OBJECTIVE
Evaluate the use of RegenPRP™ for the treatment of recalcitrant non-insertional Achilles tendinopathies and investigate whether positive outcomes depend on the age of the patients.
PROTOCOL
Retrospective study on 44 patients who had failed to respond to conservative treatment.
Patients received a total of 3 treatments at weekly intervals. RegenPRP™ (~4 ml) was injected at several sites into the degenerated tendon area. Injections were performed in sterile conditions, without anaesthesia and under ultrasound control.
Functional evaluation of Achilles tendon was performed using Victorian Institute of Sports Assessment-Achilles questionnaire (VISA-A) at baseline and at 1, 3, 6 and 12 months after the treatment. Patients were divided according to their age in two cohorts (Young <55-year-old; Elderly >55-year-old) (Fig. 1).
RESULTS
A constant and signiicant increase of the VISA-A score was observed for both patient groups during the whole length of the follow up, although better outcomes were seen in younger patients.
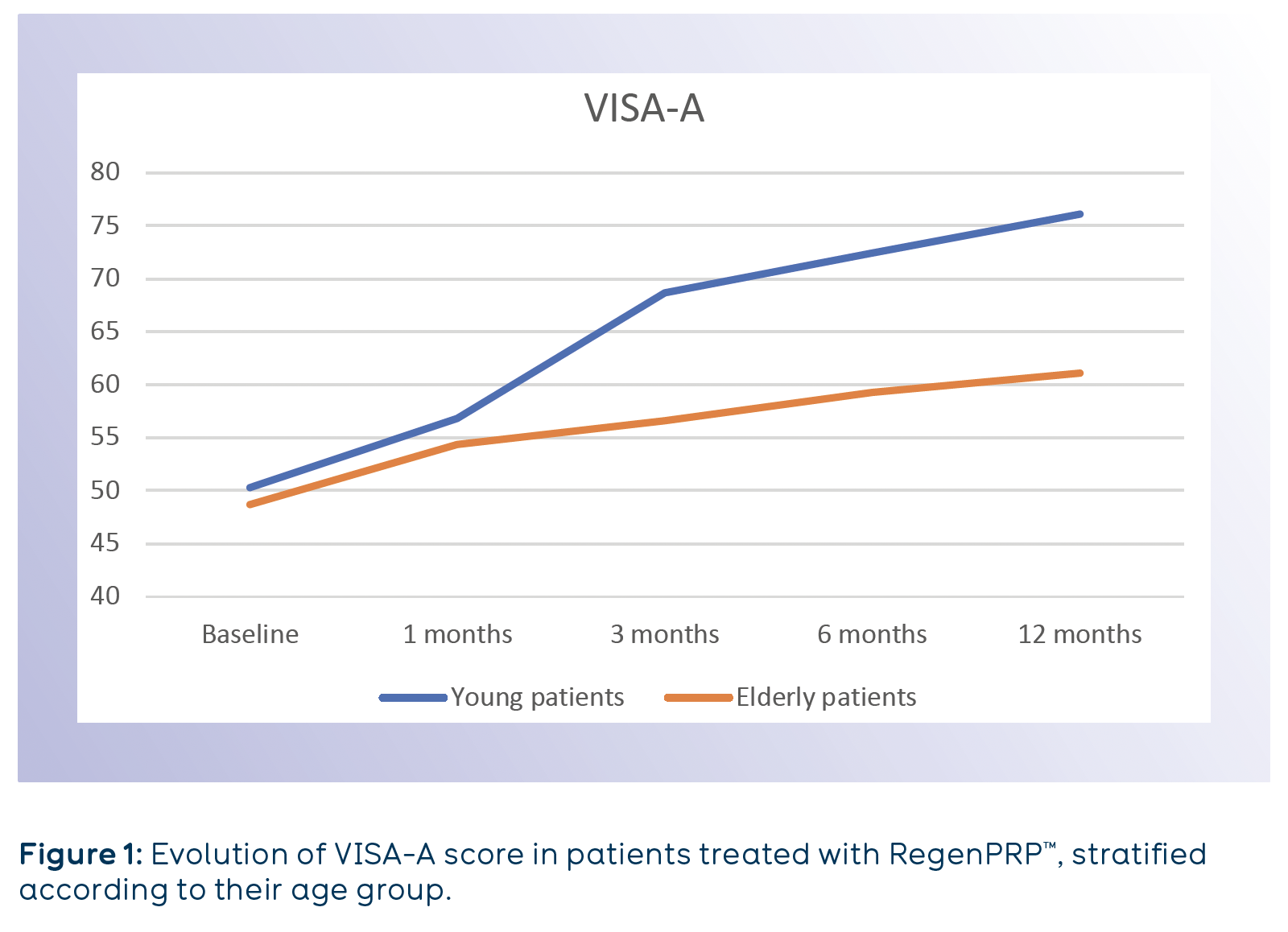
Improvement of Achilles tendon functionality after RegenPRP™ treatment was sustained for the 12 months of follow up.
CONCLUSION
When injected into tendon lesions, RegenPRP™ showed good outcomes in patients suffering from recalcitrant Achilles non-insertional tendinopathies.
REFERENCE
Salini V, Vanni D, Pantalone A, Abate Platelet Rich Plasma Therapy in Non-insertional Achilles Tendinopathy: The Eicacy is Reduced in 60-years Old People Compared to Young and Middle-Age Individuals. Frontiers in aging neuroscience 2015;7:228..
