
LICHEN SCLEROSUS
CHERNOVA 2016Lichen sclerosus (LS) is a chronic inflammatory dermatitis that creates patchy, white skin that appears thinner than normal. It usually affects the genital and anal areas. Most symptomatic patients complain of pruritus and burning or soreness, dysuria or dyspareunia.
OBJECTIVE
Evaluate the use of RegenPRP™ combined with antiviral and laser therapies for the symptomatic treatment of lichen sclerosus in patients with a concomitant papillomavirus infection.
PROTOCOL
Prospective study on 40 women with lichen sclerosus and papillomavirus infection of the vulva.
Patients were injected with 4-8 ml of RegenPRP™ in the affected area using mesotherapy technique.
The next day, antiviral Panavir gel was applied to the lesion followed by low-level laser light treatment (LLLT). Patients received 3 courses at 4-week intervals. Severity of nine symptoms were evaluated, among them itching, hypopigmentation, and oedema.
RESULTS
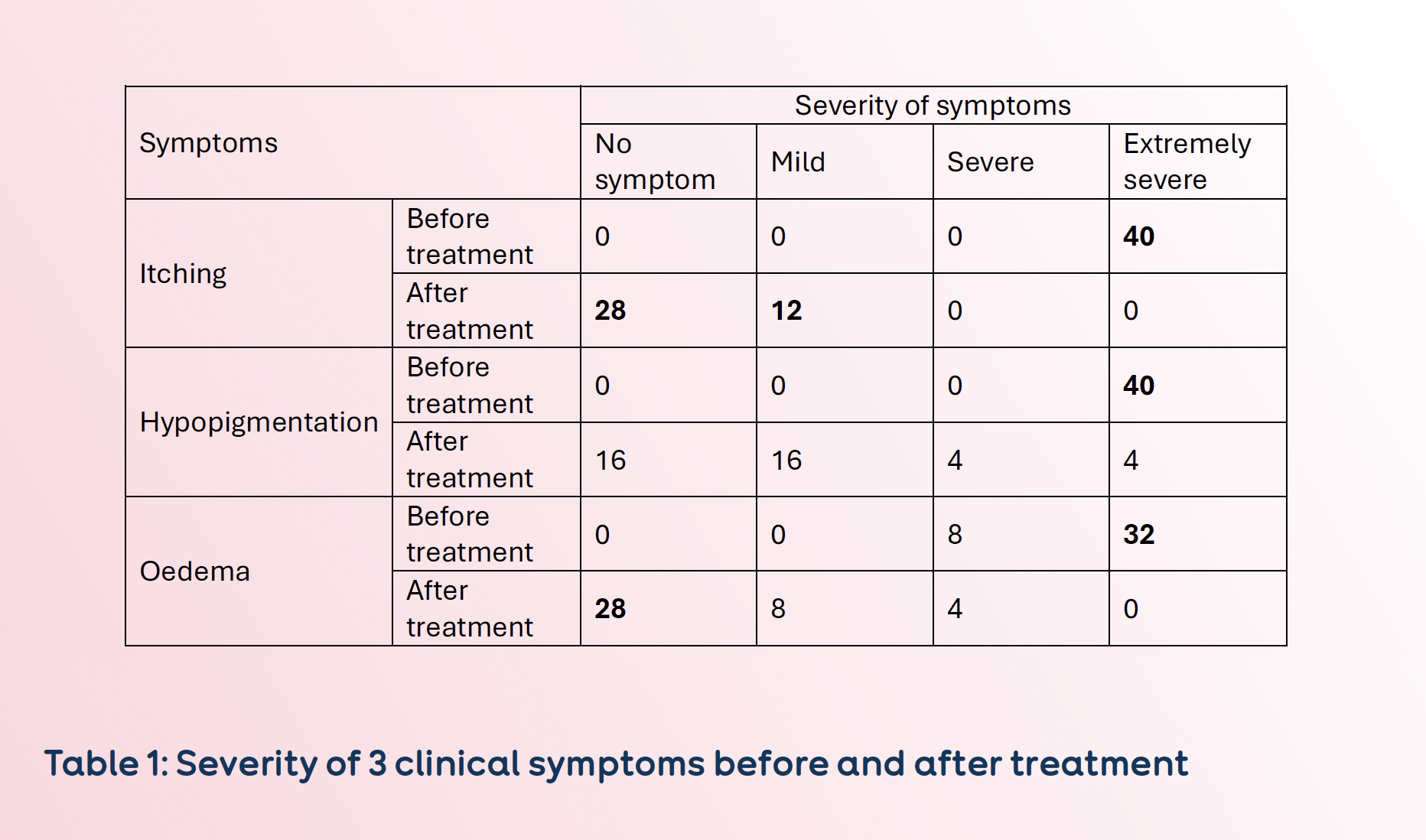
Before treatment, all 40 patients presented with extremely severe symptoms of itching and hypopigmentation; in addition, 32/40 patients presented with extremely severe symptoms of oedema (See Table 1).
One month after treatment, 12/40 patients reported only mild itching, the remaining 28 patients were without itching symptoms. The number of patients reporting severe hypopigmentation was reduced from 40 to 8, with 32 patients reporting no (16) or mild hypopigmentation (16). Twenty-eight patients reported no more oedema symptoms (See Table 1).
CONCLUSION
RegenPRP™ combined with antiviral and LLLT therapy was effective in 90% of cases (36/40) as indicated by a significant decrease in all clinical symptoms that were evaluated.
